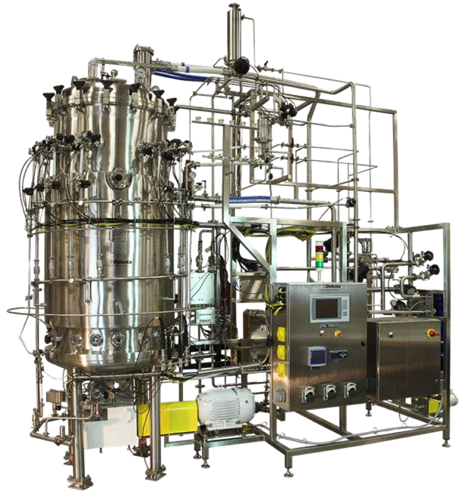
Microbial Fermentation
A Configurable and More Sustainable Fermentation Solution
Increase Your Product Output and Runtime, and Eliminate Supply Chain Challenges
Free your operations from the supply chain, and the constraints of single-use or dedicated fermentation systems. Our configurable industrial fermentation tank is easy to install into your existing framework, and all components are easy to access and maintain.
The robust, highly-reliable design enables you to produce more – and more consistently – with fewer delays and less frequent servicing.
Realize ROI With More Reliable Biotechnology System
Turn batches faster and maintain speed to market with greater flexibility in your production.
- Eliminate your reliance on the supply chain for fermentation bags, tubing, probes, and other accessories.
- Eliminate risk of bag punctures or malfunction due to potential issues during transport, setup, and use.
Our software is compatible with most distributed control systems like DeltaV and allows for quick and easy configuration to multiple product processes. Adjust mechanical settings, gas flows and agitation speeds to experiment and tailor products to your organization's needs.
Decreasing your reliance on the supply chain and enjoying greater production flexibility allows you to be swift to market, with the added benefit of being more sustainable.
By eliminating the use of single-use fermentation bags, you can adopt a more environmentally-friendly production process without sacrificing speed-to-market.
Ergonomic Biotech Equipment Solutions
Dakota Systems' biotech equipment features ergonomic design for ease of operation, durability in the working environment, and maximum productivity.
Biotech Product Lifecycle Partner
Dakota partners with our customers throughout the biotech product lifecycle. From building prototype biotech equipment to testing and validating at Alpha and Beta sites to full scale manufacturing and next generation development, we help our customers optimize product lifecycle to maximize profits and remain dominant in their space.
Single Source Biotech Equipment
Dakota Systems currently engineers and manufactures an extensive range of Biotech process-critical machinery including CIP, Filtration, Pump Skids, Chromatography, Fermentors and Cell Culture Reactors. Through compliance with current Good Manufacturing Practices (cGMP) to Final Acceptance Test (FAT), Dakota provides a comprehensive, single source BioTech Manufacturing Partner. As a single-source provider your company can rely on Dakota’s designs for repeatable results. Our documentation allows us to adapt to your needs whether you need identical machinery or if you need to develop your next-generation equipment to stay competitive in an evolving marketplace.
The Dakota Systems Difference Serves the Biotech Industry by:
- Leveraging years of experience and expertise in the ultra-high purity manufacturing industry and applying the best practices to the biotech life science industry, including life science equipment.
- Capability to deliver a fully assembled product from engineering, to welding, wiring, assembly through testing.
- Our quality team supplies documentation to comply with the validation needs of the biotech marketplace.
- Each customer is assigned a project manager giving them a single point of contact who is leading a team of industry experts to complete each project.
Dakota Systems dedicates itself to the continued improvement of the manufacturing process to improve our customers' bottom line. We strategically implement programs to help us identify the individual challenges of each of our customers and work to eliminate potential risks in their supply chain. Some of the programs implemented include Lean Manufacturing training to help our staff work strategically through each project. We also have ECO and CRM programs designed specifically to tie in with our ERP system. These programs allow us to quickly adapt to the evolving needs of our customers' next generation products or to review a customers' entire history. Each effort we make is to help our customer realize they can turn to Dakota Systems as their "go-to" partner in their supply chain.
Let your engineers and scientists focus on what they do best – delivering world class bio-pharmaceutical products, and let Dakota Systems apply our design and manufacturing expertise to deliver systems that meet and exceed your expectations.
Stainless Steel Hardwall Bioreactor Solutions
Adapt to Changing Process Requirements
Dakota Systems designs and builds bioreactor solutions with the end user and operators in mind.
Our open frame piping skid is laid out to make operation and maintenance easy. All necessary components are easily accessible by the operator.
The Dakota Lightnin/Flowserve agitators and double mechanical seals are robust and 2-3 years are typical for a maintenance cycle when using Kalrez o’rings. Dakota’s heat blanket for the exhaust filter housing are reliable and much more cost effective than an exhaust condenser. Both of these are standard offerings.
Standard systems are available in 100L, 200L, 300L, 500L, and 1000L but can be customized to meet customer requirements. Other requested sizes are optional and can be constructed up to 5000L.